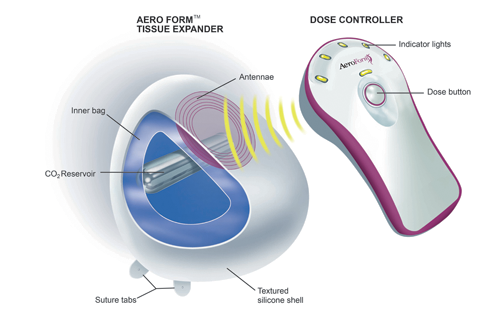
AirXpanders, Inc., a medical device company, has received US Food and Drug Administration (FDA) de novo clearance for the AeroForm Tissue Expander System, a Class II medical device used for breast reconstruction. With FDA clearance, the device can now be sold in the US, the world’s largest medical device market.
AeroForm offers a needle-free alternative for women who choose reconstructive surgery following a mastectomy. AeroForm is activated by a handheld wireless controller that administers small amounts of carbon dioxide (CO2) up to three times a day, to gradually stretch the tissue to prepare for a breast implant. With the push of a button from a remote controller, the programmed amount of CO2 is delivered in seconds, allowing the patient to continue with her daily activities while preparing for reconstruction.
AirXpanders’ president and CEO Scott Dodson said the regulatory clearance marked a major milestone and that the Company is poised to commence the commercial rollout in the United States. AeroForm is currently available in Australia, where it was approved for sale in late 2014.
“The market opportunity for AirXpanders in the US is significant, with the total addressable market worth more than US$800 million. As US mastectomy rates continue to rise and growing numbers of women undergo breast reconstruction, we are confident AeroForm will positively redefine the reconstruction process for women in the US,” said Dodson.
“While approximately 70 per cent of women who opt for reconstructive surgery undergo tissue expansion to prepare a space for breast implants, little progress has been made with regard to tissue expanders over the last 40 years,” said Jeffrey Ascherman, MD, site chief of the Division of Plastic Surgery NewYork-Presbyterian/Columbia University Medical Center, professor of surgery at Columbia University Medical Center and principal investigator for AirXpanders’ US XPAND trial.
“Reconstruction is one of the last phases of a long and sometimes taxing journey for women who are treated for breast cancer. They have lost time and control, and are eager to get back to their lives. Needle-free, patient-guided expansion could be a suitable option for many women undergoing the reconstruction process.”
AirXpanders has engaged in a range of preparatory activities to ensure it is ready for an imminent US market release, including identifying and hiring the first tier of sales personnel; dialogue with targeted hospitals for initial adoption; and conducting early industry and patient awareness initiatives.
“With the de novo FDA clearance in place, we will now accelerate the build out of our US sales force with the hiring of our direct sales team, and begin initial targeted market release in the US, similar to our successful approach in Australia,” said Dodson.
“We will first be concentrating on several key high volume academic and community hospitals that participated in our pivotal and continued access trials, as we broaden surgeon training and refine processes for seamless on-boarding with nursing, billing and inventory. Simultaneously the manufacturing transfer to Costa Rica is progressing well, where we have installed our first production line which will allow for manufacturing to commence there according to schedule.
“With this US regulatory milestone, the hiring of our initial US sales team and our continued progress with our manufacturing transfer, AirXpanders is excited to advance our efforts to bring the AeroForm to the US market.”
The company has also received an update to its existing CE Mark for AeroForm to incorporate an enhanced inner film liner which is used to contain the CO2 cannister inside of the device. This clearance allows the enhanced version of AeroForm to be sold in Europe, as well as in Australia.
Receipt of the FDA de novo clearance also enables the company to immediately file a traditional 510(k) application for an enhanced design that has been incorporated in the latest version of the CE-marked AeroForm, as it will be sold in Australia. FDA clearance for that product enhancement is anticipated in 2QCY17, which will precede full scale US commercial launch.
Founded in 2005, AirXpanders, Inc. designs, manufactures and markets innovative medical devices to improve breast reconstruction. The company’s flagship product, the AeroForm Tissue Expander System, is used in patients undergoing two-stage breast reconstruction following mastectomy.
Us fda grants, de novo clearance to airxpanders, aeroform tissue expander system, breast reconstruction